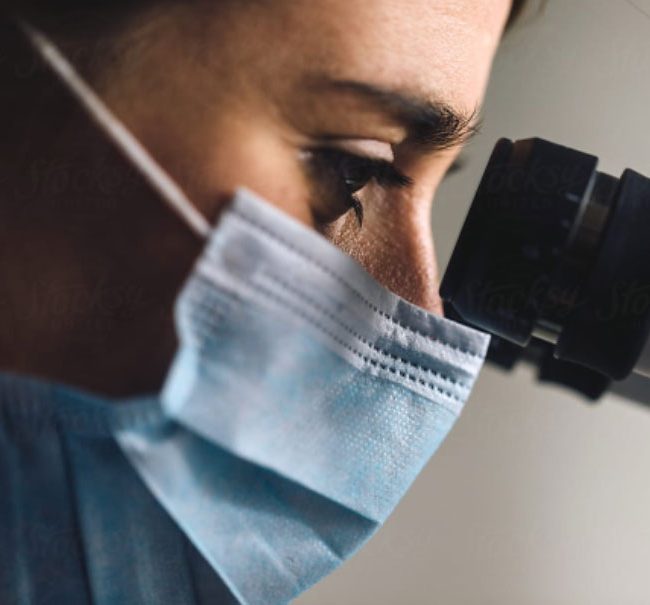
Medical Device Single Audit Program (MDSAP)
If you are a medical device manufacturer, take advantage of the MDSAP.
This unique program for Medical Devices allows you to apply for a single assessment of your quality management system (QMS) valid for all five participating countries: the United States, Canada, Brazil, Australia, and Japan.
Sections
The Certification Process
- Gathering of information from the applicant manufacturer
- Analysis of information in relation to the scope of GMED’s recognition
- Initial audit preparation and planning
- Audit
- Issuance of interim report to the company
- Submission of action plan by the company, if needed
- Delivery of action plan analysis
- Finalization of audit report and transmission to the company
- Surveillance audit: 1st year
- Surveillance audit: 2nd year

About the MDSAP program
In 2012, the International Medical Device Regulators Forum (IMDRF) recognized the value in developing a global approach to auditing and monitoring medical device manufacturing in order to ensure medical device safety. A Medical Devices Single Audit Program was launched for the primary purpose of “jointly leveraging regulatory resources to manage an efficient, effective and sustainable single audit program focused on the oversight of medical device manufacturers.”
When using the MDSAP, a medical device manufacturer enlists a recognized Auditing Organization (AO) to conduct the audit of its QMS based on the regulations of the five participating countries: Australia, Brazil, Canada, Japan, and the United States.
Under the MDSAP, the manufacturer’s quality management system undergoes a single assessment every year, with a full certification cycle of three years. This considerably reduces the number of audits and inspections that the medical device manufacturer must undergo with national regulators.
Each country decides how the MDSAP results apply in accordance with its laws. To date, the only country that has made the MDSAP mandatory is Canada, where it has been a requirement for class II, III, and IV medical devices (MDs) since January 1st, 2019.
The MDSAP audit results (audit report) are systematically uploaded to a database that can be accessed by all participating regulatory authorities.
The MDSAP takes a global approach to quality management system audits and operates as an international coalition of countries dedicated to pooling resources, technologies, and services in order to improve safety and surveillance of medical devices around the world. The MDSAP is useful for medical device manufacturers who want to market their devices in the five countries that participate in the program.
MDSAP Benefits
- Optimization of regulatory resources in one program to market your medical devices internationally in up to five countries: Japan, Brazil, Canada, Australia, and the United States;
- ISO 13485:2016 certification for your company’s QMS compliance through the MDSAP;
- Participation in an evolving international program, with 2 new affiliate members recently joining: South Korea and Argentina.
The MDSAP is voluntary in Australia, Brazil, Japan, and the United States. However, it has been mandatory in Canada for class II, III, and IV medical devices since January 1st, 2019.
When manufacturers decide to participate in the MDSAP, they must list, in the certification contract, the countries where they are and/or will be marketing their medical devices. During the on-site audit, the manufacturer’s QMS compliance is reviewed against the national regulations of those countries
Medical device manufacturers must list the countries where they market their product in their certification contract.
The MDSAP audit criteria include the provisions of ISO 13485, as well as the related requirements of participating regulatory authorities. In addition, other specific requirements from each regulatory authority may apply to products, licenses, company registrations, adverse event reports, etc.
An important provision for those implementing the MDSAP is that manufacturers may exclude the requirements of a country in which they have no intention to market medical devices. This means that the MDSAP audit criteria include ISO 13485 provisions and only the regulatory requirements that apply in the MDSAP countries where the company sells or intends to sell its product.
CE marking certification is independent from MDSAP certification. The European Union has an observer status and has no role in implementing the MDSAP. Both certification programs, however, can be conducted jointly.
This special status, created in 2019, allows countries to use MDSAP certificates or audit reports to evaluate medical device manufacturers’ quality management systems within their own regulations.
As of the time of writing (March 2020), South Korea (Ministry of Food and Drug Safety) and Argentina (ANMAT) are the only affiliate members.
Participating Countries
United States
The Center for Devices and Radiological Health (CDRH), a branch of the FDA, accepts MDSAP audit reports as a substitute for FDA routine inspections (every two years, according to established procedures).
Exceptions
- Inspections conducted “For Cause” or as “Compliance Follow-up”;
- Necessary pre-approval or post-approval inspections for Premarket Approval (PMA) applications; decisions under section 513(f)(5) of the Act (21 U.S.C. 360c(f)(5)) concerning the classification of a device;
- Combination products.

Choose GMED to take advantage of the MDSAP
- GMED is recognized as an Auditing Organization by the MDSAP Regulatory Authority Council (RAC). It has participated in the program since it was launched by authorities, and has been conducting MDSAP audits since April 2015;
- Not only does GMED have a solid reputation, but most of its auditors are qualified to conduct both Quality Management System audits (MDSAP and ISO) and regulatory audits (CE marking), an added benefit for medical device manufacturers wishing to combine their certification processes;
- When you combine your certification processes, you can rest assured that GMED, as your Auditing Organization, will provide you with optimized planning, reducing the time your company’s resources have to devote to the audit cycle.
News
View all our technical, regulatory and normative information including guides, webinars, news…
Combining Certifications
Request for MDSAP Certification
With offices in Europe and North America, we serve the medical devices industry worldwide.
