Our expertise enhancing your skills
As a professional in the medical device or in vitro diagnostic medical device industry, you want to develop the skills and knowledge you need to handle the challenges you’re facing.

Choose GMED North America’s Trainings
Established international organization
GMED is a notified body and an international certification body for products and quality management system in the medical device field.
Our experts’ experience and knowledge of the medical device industry allow us to offer a variety of training content adapted to the medical device manufacturers’ needs.
We focus on your challenges and provide attendees with a clear “take-home” value.
GMED’s trainings feature
- Hands-on practical case studies to encourage material retention & application;
- Qualified Trainers & Medical Device Experts for a gain of experience;
- In-house and/or public training scheduled for your convenience.
Limited number of attendees per session
The number of participants is maximized to fifteen to provide a stimulating and practical working environment.
We help you built-in knowledge checks that will allow you to apply and reinforce the mastery of new concepts explored throughout the training.
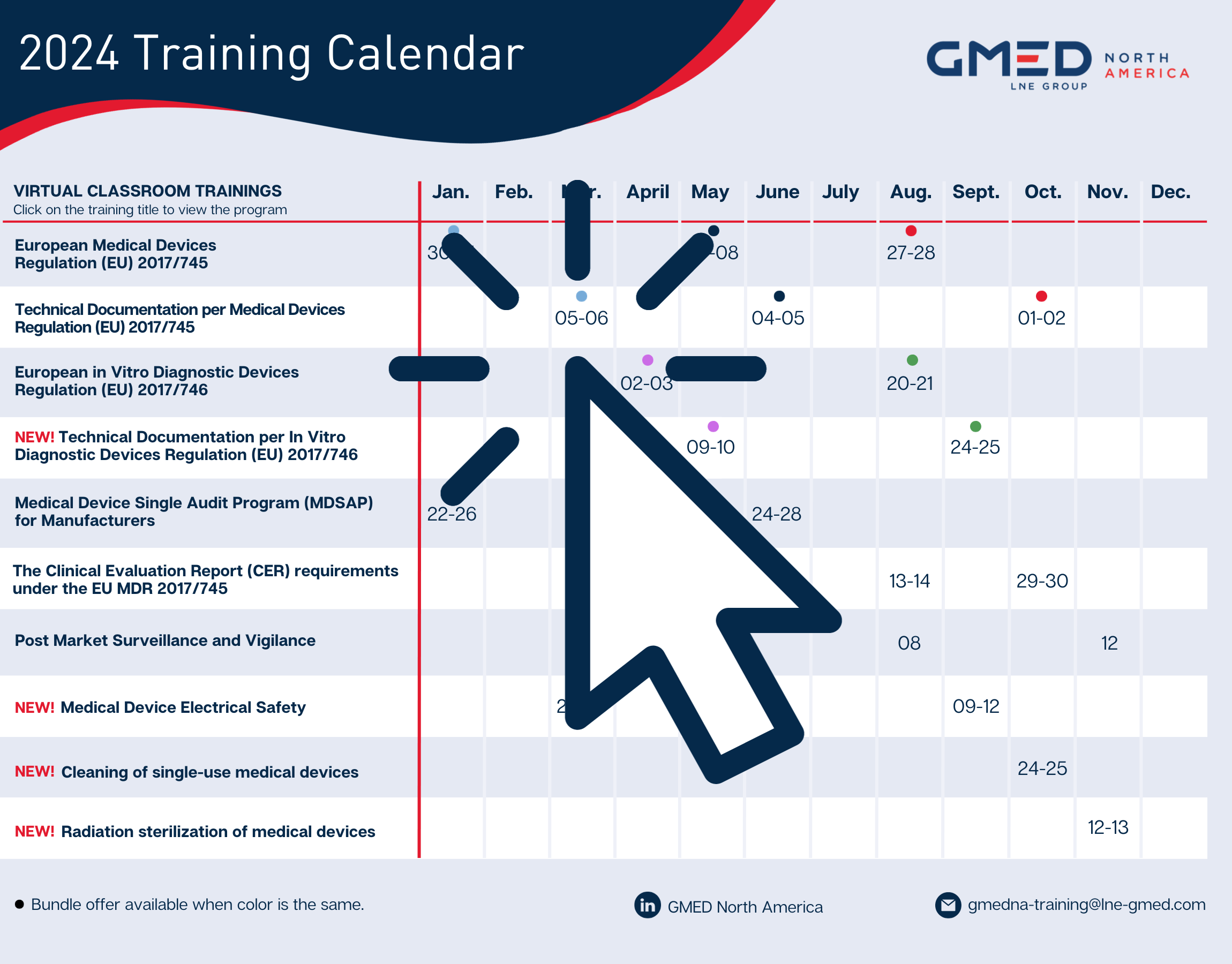
2024 training calendar
Our wide range of virtual training courses will enable you to acquire the skills you need to implement European and international regulations and manage risks.
Download the 2024 training calendar by clicking on the icon, and book the course(s) that will enable you to meet the technical, regulatory, or normative challenges you are facing.
Training Catalog
Cleaning of Single-Use Medical Devices
[NEW in 2024]
Clinical Investigation of Medical Devices
[NEW in 2024]
European in Vitro Diagnostic Devices Regulation (EU) 2017/746
European Medical Devices Regulation (EU) 2017/745
ISO 13485:2016 Requirements
Medical Device Electrical Safety
[NEW in 2024]
Medical Device Single Audit Program (MDSAP) for Manufacturers
Medical Device Software Lifecycle per IEC 62304:2006
[NEW in 2024]
Post Market Surveillance and Vigilance
Request a training today
We offer a variety of training courses and focus on your challenges to provide our attendees with a clear “take-home” value