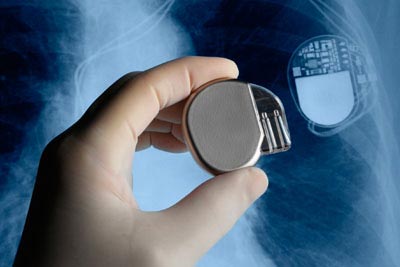
 High tech devices, such as pacemakers, cochlear implants and radioactive brachytherapy seeds, can have a dramatic impact on patient care.
High tech devices, such as pacemakers, cochlear implants and radioactive brachytherapy seeds, can have a dramatic impact on patient care.
Given the surgical procedure involved and integration of a source of energy (electrical or not), such devices have to go through an intense amount of scrutiny to assure safety.
Challenges
Devices covered by the Active Implantable Medical Devices (AIMD) rules are treated as Class 3 devices which require a thorough assessment procedure. AIMDs calls upon the general requirements of harmonized standard EN 45502 as well as requirements to a specific family of device types.
Not all Notified Bodies are recognized under directive 90/385/EEC (revised by the directive 2007/47/EC) to certify such devices.


 We're Hiring!
We're Hiring!